| Surgical Face Mask | Surgical Face Mask (Umbrella EUA) | General Face Mask (During Public Health Emergency) |
General Face Mask (During Public Health Emergency) |
KN95 Respirator (During Public Health Emergency) |
KN95 Respirator (During Public Health Emergency) |
N95 Repirator | |
| FDA Product Code | FXX | QKR | QKR | QKR | QKR | QKR | FXX |
| Classification Name | Mask, Surgical | Enforcement Discretion | Enforcement Discretion | Enforcement Discretion | Enforcement Discretion | Enforcement Discretion | Mask, Surgical |
| Regulation Number | 878.404 | N/A | N/A | N/A | N/A | N/A | 878.404 |
| Premarket Notification | 510K | N/A | N/A | N/A | N/A | N/A | Exempt |
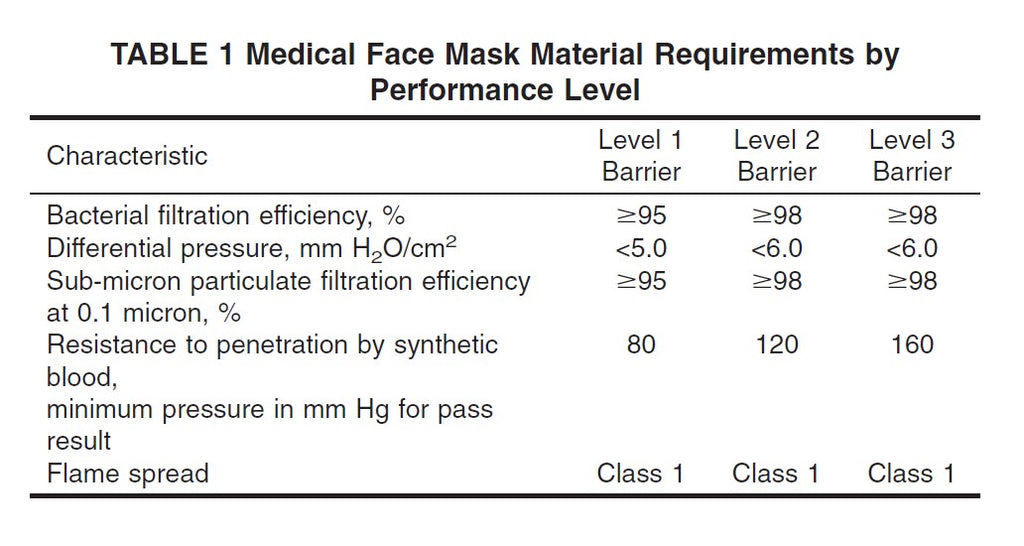
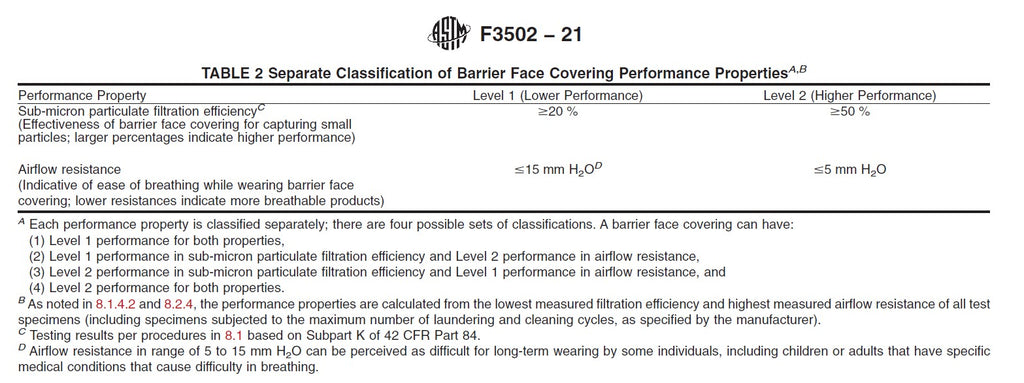
| US Standards | ASTM F2100-19 | ASTM F2100-19 | ASTM F2100-19 | ASTM F3502-21 | ASTM F3502-21 | ASTM F3502-21 | NIOSH |
| China Standards | N/A | N/A | N/A | N/A | GB2626-2019 | GB2626-2019 | N/A |
| Wearing Type | Earloop/Tie-On | Earloop/Tie-On | Earloop/Tie-On | Earloop/Tie-On | Earloop | Headband | Headband |
| Layer | 3ply/4ply | 3ply/4ply | 3ply/4ply | 3ply/4ply | 5ply | 5ply | 5ply |
| Shape | Flat | Flat | Flat | Flat | 3D | 3D | 3D |
| Best Performance | Level 3 Barrier* | Level 3 Barrier | Level 3 Barrier | Level 2** (Higher Performance) |
Level 2 (Higher Performance) |
Level 2 (Higher Performance) |
N95 |
| Protection Rank | 4 | 5 | 6 | 7 | 3 | 2 | 1 |
| Cost-Effective Rank | 2 | 2 | 1 | 3 | 4 | 5 | 6 |
Tips:
Headband is better than Earloop
4ply is better than 3ply (Flatmask only)
QKR Defination
Face mask is intended to be worn by general public or healthcare personnel and excludes air purifying respirators. The mask covers the user's nose and mouth and may or may not meet fluid barrier or filtration efficiency levels. The device is either subject to the Enforcement Policy for Face Masks, Surgical Masks, Barrier Face Coverings, Face Shields, Surgical Masks, and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency, available at FDA Enforcement Policy, provided that the device does not create undue risk by following the recommendations for this device type outlined in the guidance, or to the Face Mask Umbrella EUA, available at FDA.